sahana pharmaceuticals
Quality Management
License number 3726 under Form 25 and 3727 under Form 28.
Issued by Director of Drugs Control
Quality control
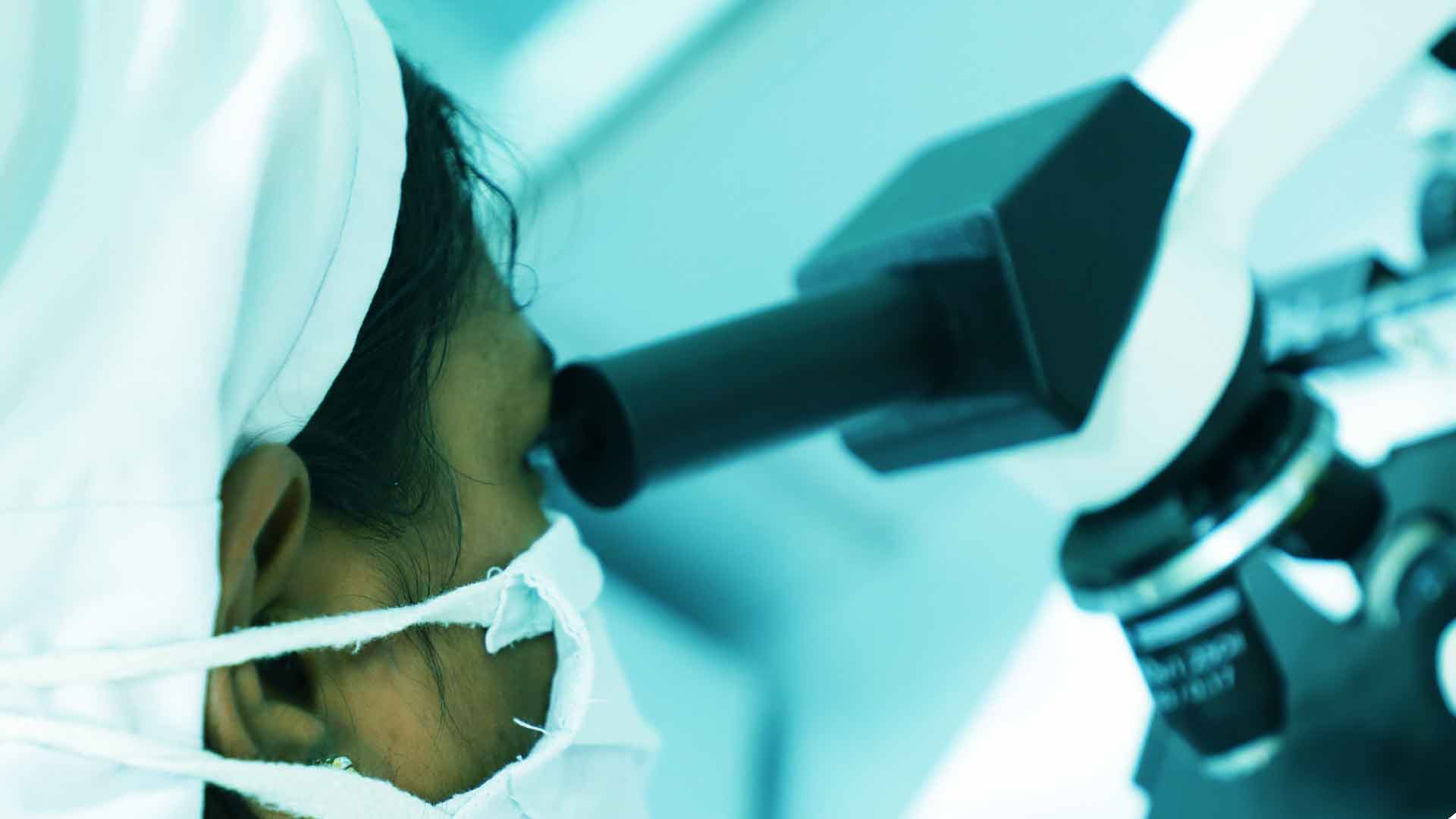
Our Quality Control department has experienced competent and technically qualified personnel and quality control head to shoulder various activities of the department. Our laboratory has the facilities of chemical instrumental, microbiological and stability testing. Instrumental room is temperature controlled.
The chemical laboratory has a well-laid Standard Operation Procedure(SOPs) ,in Instrumentation laboratory was highly equipped with a latest equid like HPLCs, UVs, among other instruments.
Our instruments used in analytical purpose are operated and calibrated as per the respective operating and calibrating procedures.
All the working standards used here are carefully selected and analyzed. Working standards are qualified using Proper Reference standards.
Quality Management
We are following approved Standard operating procedures for each and every activity carried out at the site. The plans of the procedure is the responsibility of the user, and a trained team give a positive assurance about the product based on their observation
The manager QA approve everything before the checking of reports, protocol and analytical limits. And he also approve the specification of raw material and packing material
The quality assurance department analyzed about the finished product, review of the batch manufacturing and packing records after the formalities they release the product for sale or distribute.
Micro-biology
Micro-biology
The main purpose of our lab is assisting to diagnosis the infectious diseases. In our laboratory, we have a specially trained staff mainly for microbiology and infectious disease. And we occur a number of steps to detect your disease, we collect a specimens like blood urine and swab specimens of the throat. . A good quality specimen helps to diagnosing the infectious disease. Finally the technology detects the organism. And we announce the result from 15 minutes to eight weeks. Our microbiology laboratory leads a good relationship and good communication with the clinician and infection control practitioner.
Quality Management
Quality Control
Our Quality Control department has experienced competent and technically qualified personnel and quality control head to shoulder various activities of the department. Our laboratory has been designed and equipped with facilities for chemical, instrumental, microbiological and stability testing. Instrumental room is temperature controlled.Our instruments used in analytical purpose are operated and calibrated as per the respective operating and calibrating procedures
Material Receipt & Labeling
Materials are approved only from the approved vendors. Dispensing of materials is a controlled operation carried out by stores personnel in the presence of production and quality assurance personnel. Line Clearance procedures are followed for all manufacturing and packing operations
Documentation
Our site is engaged with a well-defined system of Document Control. There is an approved procedure for the system of document preparation, revision, distribution, storage and destruction of the obsolete documents
Quality Assurance
Internal audits are conducted at the site at defined intervals. The inspection reports and compliance reports are documented by factory quality assurance
About Sahana
Sahana Pharmaceuticals commissioned in the year of 2014 at the heart of Kanyakumari district. We are engaged in manufacturing and marketing varied formulations of Tablets in 2015. Sahana strives to provide high-quality pharmaceutical products that improve the health of the patients.
Location
SAHANA PHARMACEUTICALS (P) LTD
No :20/72/4 Main Road,
Melakattuvilai Maniketipottal (P.O)
Kanyakumari (Dist) Pin- 629501
(+91) 9488887794
+914652-286661,
Products
Newsletter
Get Social
© Copyright 2019 Sahana Pharmaceuticals Pvt Ltd.
All rights reserved & Powered by LezDo TechMed